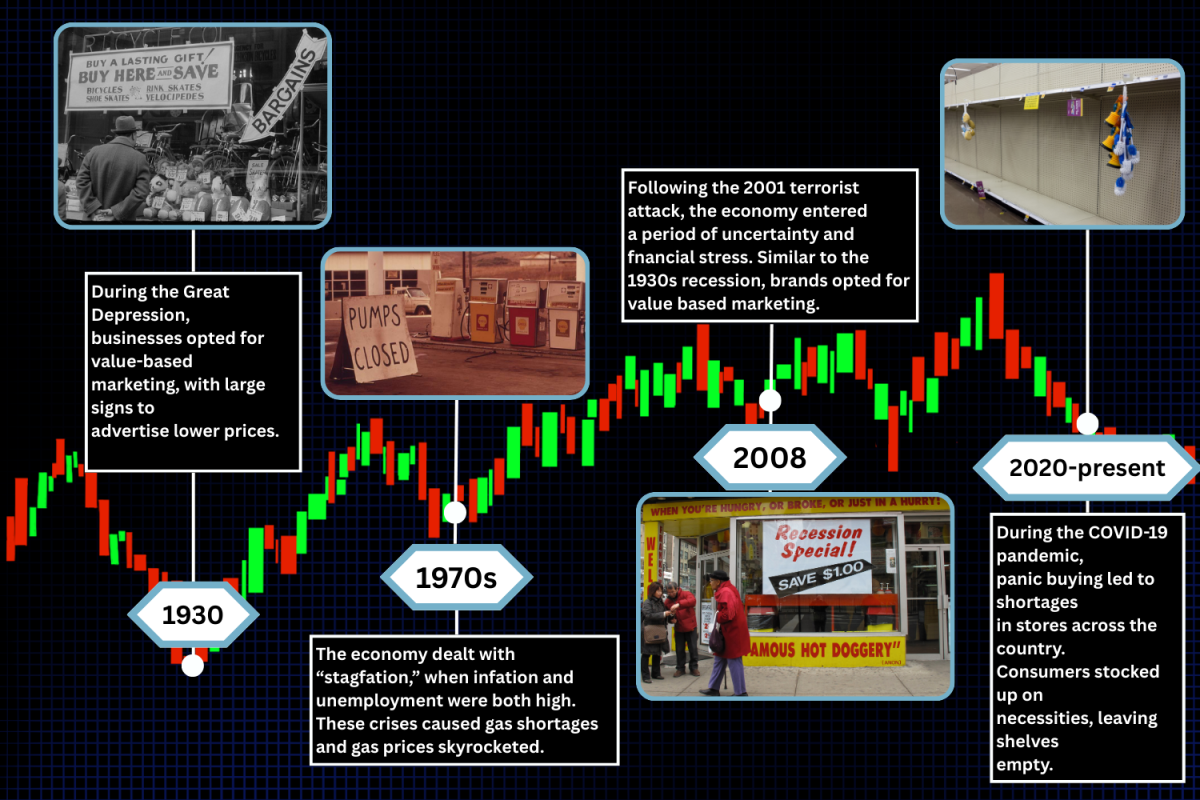
FDA drug approval fails to protect public health
New painkiller tablet Dsuvia is 500 to 1000 times stronger than morphine
Food and Drug Administration’s drug approval fails to protect public health
December 14, 2018
When a drug is approved by the Food and Drug Administration (FDA), it is typically perceived as safe to consume. After all, its approval means that the drug has been determined by the federal government to provide benefits that outweigh its known or potential risks for the intended population. Such risks can include serious adverse reactions when the drug is taken, toxicity and misuse of the drug. According to the Centers for Disease Control and Prevention, around 48.5 million Americans reported use of illicit drugs or misuse of prescription drugs in 2016. Despite this knowledge, on Nov. 2, the FDA approved Dsuvia, an opioid 500 to 1000 times stronger than morphine. This approval reminds individuals that it is important to take the responsibility to conduct thorough research and consult healthcare professionals to weigh the pros and cons of what is entering their bodies before consumption.
Dsuvia is a tablet form of sufentanil, a highly addictive painkiller. Its main use is to provide instant relief for moderate-to-severe pain in a hospital setting when alternative treatments are inadequate. After receiving the FDA’s approval, Dsuvia’s launch into the market is expected in the first quarter of 2019.
“Since Dsuvia is a tablet, I think that it is a lot easier for people [to take the drug],” said senior Samiksha Patil, co-president of Neuroscience Club. “However, [the FDA] needs to regulate the drug closely, because it can easily become heavily abused.”
The FDA is responsible for protecting public health, including ensuring the safety of the nation’s food supply and cosmetics, as well as the safety and security of biological products and human and veterinary drugs. During its drug approval process, the FDA reviews laboratory, animal and human clinical test results to decide on whether the drug is considered safe and effective for consumption. This is determined based on clinical endpoints, outcomes that represent direct clinical benefits such as decreased pain, which are presented by data from the test results. However, the clinical tests are not conducted by the FDA but by pharmaceutical companies, who send test results to the FDA for approval. Thus, critics have voiced concern that some of the drug research submitted by the pharmaceutical companies may potentially be biased and ambiguous, making test results appear as if the benefits of the drug outweigh any risks to patients using them, even if that might not be the case.
In 1992, the FDA introduced its Accelerated-Approval Program to allow for earlier approval `of drugs intended to treat serious or life-threatening conditions. For such drugs, the FDA determines the efficacy of the drug based on the results of a surrogate endpoint rather than a clinical endpoint. Surrogate endpoints are measures of effect of a specific treatment that are used to substitute clinical endpoints. They expedite the approval process as they are designed to be easier and quicker to measure than clinical endpoints which are much more specific. However, unlike clinical endpoints that have a direct relationship with the drug’s clinical benefits, surrogate endpoints only suggest a relationship such as tumor shrinkages can predict longer survival. This means that it is possible for a drug that influences a surrogate measure to have no meaningful effect on the patient. Following the FDA’s introduction of its accelerated process, a study conducted by JAMA Internal Medicine showed that from 2003 to 2012, 31 out of 36 studied cancer drug approvals that were made on the basis of a surrogate end point had unknown effects on overall survival or failed to show gains in survival, suggesting that in only a few cases did surrogate measures predict actual benefits for patients.
“It’s important to have a slower approval rate, because even though it’s slower, it’ll be [more] effective,” said sophomore Caitlyn Wong, who used a prescribed anesthesia after undergoing two surgeries on her right lower leg, most recently in August 2017. “If [the FDA] has a drug that’s approved, didn’t find out the drug’s possible side effects and a lot of people are using it and being harmed by it, then it could have a really bad effect on the population. Also, if that drug is FDA approved, [the FDA] would lose its credibility.”
According to the National Institute on Drug Abuse (NIDA), opioid overdoses increased 30 percent from July 2016 through September 2017 in 45 states. With an opioid epidemic in the U.S. on the rise, Dsuvia has sparked controversy. Those who oppose Dsuvia assert that the newly FDA-approved drug would only worsen the opioid epidemic due to its potential misuse. On the contrary, supporters argue that close monitoring, such as tightly controlling Dsuvia’s distribution and use, would limit potential abuse. Supporters also believe that the FDA’s plan for Dsuvia to be used strictly in certified medically-supervised healthcare settings, such as surgical centers and emergency departments, would further prevent possible abuse of the drug.
“Knowing the FDA, it probably consulted many health professionals,” said chemistry teacher Lester Leung. “This approval does not that mean that the Dsuvia can be used in homes, so it’s not just that [Dsuvia] can be used in any situation. So, with that, I trust the FDA decision, the safeguards that the FDA put in place and all the procedures that it followed to make this decision.”
Although the FDA intends to monitor Dsuvia closely and asserts that Dsuvia should not be used for more than 72 hours, this has not prevented widespread alarm. The opioid epidemic began in the late 1990s, when opioid medications were prescribed in greater rates since pharmaceutical companies had assured consumers that opioid pain relievers would not cause addiction. Furthermore, according to the NIDA, more than 115 people died each day in the U.S. in 2018 due to opioid overdose, in comparison to an average of 83 deaths each day in 2015, which shows a recent increase in opioid overdoses. In the midst of an opioid epidemic, introducing another potent opioid drug would only exacerbate the problem. When Dsuvia is placed into the market, there is the risk that users might develop an opioid addiction, since opioids activate powerful reward centers in the brain by boosting dopamine levels, which can give people a high. The body can develop a tolerance that can lead into an addiction in which the more painkillers consumed, the larger dose is needed for the drug for the user to reach the same high.
“It’ll start off where [the drug] is a pain medicine. So, you’ll take it, and it will pacify the pain a bit,” said junior Shubhra Dubey, who took the painkiller Vicodin after undergoing surgery when she tore a ligament in her knee in August. “But eventually, your brain will start off like, ‘it kind of hurts, maybe I should take a pill or something.’ My mom was controlling how much I took, so I didn’t really take the pills that often, but I can see how people could get addicted.”
The controversy surrounding Dsuvia brings more attention to the FDA drug-approval process. Although the FDA’s accelerated-approval regulatory process expedites the approval of drugs used to treat serious conditions, it also gives the FDA less time to consider the benefits and risks of a new drug, which can cause the agency to overlook possible safety concerns. Given this, it is even more important to consult doctors and other healthcare professionals regarding the pros and cons of a drug and determine whether or not a specific drug is really safe to consume.